health
Mpox and the challenges of global health governance

In the realm of global health, the recent developments surrounding Mpox have once again brought to light the complex challenges associated with global health governance. The handling of Mpox, a viral infection that has plagued communities worldwide, raises questions about the effectiveness of international cooperation, the role of governing bodies, and the need for coordinated response strategies. Understanding these challenges is crucial to strengthening global health systems and improving our collective ability to respond to future health crises.
Mpox, a highly contagious viral disease, has been a persistent global health concern for decades. Its rapid spread and potential for severe complications demand a coordinated and united response from the international community. However, the governance of global health poses several obstacles that hinder effective and timely action.
One of the key challenges lies in the fragmented nature of global health governance. Various organizations, including the World Health Organization (WHO), United Nations agencies, regional bodies, and national governments, play roles in shaping and implementing global health policies. The multiplicity of actors, each with its own priorities and interests, often leads to a lack of coordination and cohesive decision-making.
Dr. Amanda Roberts, a global health policy expert, explains, “The governance of global health is a complex web of actors, with overlapping mandates and limited coordination mechanisms. This fragmentation can impede swift and unified responses to global health threats like Mpox.”
The Mpox crisis has highlighted the need for stronger coordination among global health entities. It has prompted discussions on the establishment of mechanisms that facilitate collaboration, information sharing, and joint decision-making. Enhancing the role of the WHO as a central coordinating body and fostering greater cooperation between governments, civil society, and private sector stakeholders are crucial steps in overcoming these challenges.
Another challenge in global health governance is the issue of compliance and enforcement. While international health regulations and guidelines exist, ensuring their implementation and enforcement across countries remains a significant hurdle. Disparities in resources, capacity, and political will can impede the effective implementation of global health measures, leaving gaps in preparedness and response efforts.
The Mpox outbreak has underscored the need for a robust system of compliance and accountability. Dr. James Anderson, a public health expert, emphasizes, “To effectively address global health threats like Mpox, we need a system that not only sets standards but also monitors compliance and holds countries accountable for their actions.”
Strengthening mechanisms for monitoring and evaluation, as well as promoting transparency and information sharing, can enhance compliance with global health regulations. This requires concerted efforts from governments, international organizations, and civil society to ensure that health policies are implemented consistently and effectively.
Furthermore, the financing of global health initiatives presents a significant challenge. Adequate and sustainable funding is essential for building resilient health systems, supporting research and development, and ensuring equitable access to healthcare. However, funding for global health programs often falls short of the required levels, leading to gaps in preparedness, response, and healthcare delivery.
The Mpox crisis has highlighted the urgent need for increased investment in global health. Dr. Lisa Roberts, a health economist, explains, “Insufficient funding for global health not only hampers our ability to address immediate health crises like Mpox but also undermines long-term health security and resilience.”
International cooperation and innovative financing mechanisms, such as public-private partnerships and global health funds, can help bridge the financing gap. Additionally, advocating for increased domestic investments in health and addressing systemic issues that perpetuate health inequalities are vital steps toward achieving sustainable and equitable healthcare systems.
In conclusion, the Mpox crisis has shed light on the multifaceted challenges associated with global health governance. The fragmented nature of global health governance, issues of compliance and enforcement, and inadequate financing pose significant hurdles to effective response and prevention efforts. Addressing these challenges requires collective
action, political will, and a commitment to strengthening global health systems.
As the world navigates through the Mpox crisis and prepares for future health challenges, it is essential for governments, international organizations, and civil society to come together, strengthen coordination mechanisms, ensure compliance with global health regulations, and increase investment in health. Only through collaborative and proactive efforts can we build a resilient and inclusive global health governance framework that safeguards the well-being of individuals and communities worldwide.
health
Long-Term Asthma Management: Safeguarding Lung Health

Breathing easily today is vital, but ensuring that you can breathe well for the next ten years is even more essential. This is where the expertise of an Asthma Specialist becomes invaluable. Asthma isn’t merely about managing occasional symptoms; it fundamentally involves safeguarding your lungs for a lifetime.
Many people tend to seek medical assistance only when their symptoms flare up. However, maintaining long-term respiratory health relies on consistent care, timely adjustments, and a deep understanding of how your lungs change over time.

Asthma: A Long-Term Condition
Asthma is not a fleeting illness that disappears once symptoms lessen. Even during seemingly “good phases,” inflammation may still be silently active, posing risks to lung health. An Asthma Specialist looks beyond immediate symptom relief and emphasizes long-term lung preservation.
This proactive approach can help prevent a gradual decline in lung capacity that often goes unnoticed until everyday activities become increasingly challenging. Regular check-ups and ongoing monitoring are essential. These enable specialists to evaluate lung function and adjust treatment plans accordingly.
Engaging in proactive management strategies, such as making lifestyle changes and adhering to prescribed medications, is crucial for effective asthma control. Additionally, educating patients about potential triggers and avoidance strategies equips them to manage their condition more effectively.
The Significance of Long-Term Monitoring
Over time, the triggers for asthma may transform. Stress levels fluctuate, and work environments change. A proficient Asthma Specialist monitors these variations and tailors treatment as necessary. What worked two years ago may no longer be sufficient, and routine follow-ups help catch issues early.
Picture this process like maintaining a car: regular service prevents breakdowns rather than merely addressing problems after they occur.
Personalized Care: A Tailored Approach
Every asthma patient is unique, influenced by factors like lifestyle, allergies, sleep patterns, and emotional well-being. An Asthma Specialist envisions a personalized care plan that fits into the patient’s real life rather than disrupting it.
This tailored approach enhances adherence to treatment and proves far more effective in the long run.
Preventing Flare-Ups: A Proactive Strategy
Emergency visits frequently occur when subtle warning signs are overlooked. An Asthma Specialist educates patients to recognize early changes in their breathing and take action before symptoms escalate.
By implementing this proactive strategy, patients can reduce hospital visits and missed workdays, alleviating unnecessary anxiety.
Education: Core to Long-Term Care
One often-overlooked role of an Asthma Specialist is the emphasis on education. By teaching patients the correct inhaler technique, trigger avoidance, and how to track symptoms, specialists empower individuals to take control of their asthma management.
Patients who have a thorough understanding of their condition tend to experience fewer flare-ups and better overall control.
The Importance of Expertise in Chronic Asthma Care
Effective long-term asthma management demands experience rather than guesswork. Specialists like Dr. Sanchayan Roy are known for aiding patients in achieving stable lung health over years, rather than only addressing symptoms as they arise.
Experience enables healthcare providers to recognize subtle changes before they escalate into serious problems.
Patient Experiences Post-Diagnosis
Many patients express a common realization: “I didn’t know breathing could feel this effortless.” That moment of clarity highlights why Asthma Specialists look beyond mere symptoms.
When breathing improves, it positively impacts other life areas—energy levels rise, focus sharpens, and confidence grows.
Early Identification: Minimizing Long-Term Damage
If asthma goes undetected, lingering inflammation can silently continue to affect the airways. Over time, this may lead to lasting damage. An Asthma Specialist aims to intervene early when treatment is more straightforward and lung function is recoverable.
Taking early action protects future breathing capabilities.
Choosing the Right Care: The Need for Expertise
Not all breathing issues are readily identifiable. Consulting with the Best Asthma Doctor in Delhi ensures access to advanced diagnostic tools, clinical expertise, and ongoing monitoring. A skilled Asthma Specialist doesn’t rush to conclusions; rather, they approach each case with meticulous care.
This level of detailed attention can often prevent years of unnecessary discomfort.
Urban Living: Managing Long-Term Lung Health
Living in urban environments exposes individuals to pollution, allergens, and stress. An Asthma Specialist tailors treatment plans to address these urban challenges, minimizing the cumulative impact of triggers on lung health.
This tailored guidance is particularly important for those who notice a gradual decline in their breathing over time.
The Benefits of Ongoing Specialist Care
Patients frequently report that they were unaware of how restricted their breathing had become until improvements were made. An Asthma Specialist focuses on restoring comfort, stamina, and confidence—not merely managing attacks.
Long-term care isn’t about limitations; it’s about achieving freedom in daily life.
The Best Asthma Doctor in Delhi: Choosing the Right Partner
For long-lasting respiratory health, many patients search for the Best Asthma Doctor in Delhi—someone who provides continuity, advanced diagnostics, and a strategic vision for lung health. The right Asthma Specialist becomes a partner in health rather than just a consultant.
Prioritizing Future Lung Health Starts Today
Asthma management isn’t solely about immediate relief. An Asthma Specialist strives to ensure that your lungs remain robust, flexible, and functional for years to come, eliminating the need to constantly think about breathing.
Conclusion
Long-term respiratory health is not a matter of luck; it is cultivated through informed care, vigilant monitoring, and expert guidance. The role of an Asthma Specialist transcends symptom control; it is fundamentally about protecting lung function for life. With a patient-centered approach and support from the Best Asthma Doctor in Delhi, managing asthma becomes more predictable and far less intrusive. Learn how an Asthma Specialist in your area can facilitate long-term respiratory health through early intervention, comprehensive monitoring, and personalized care.
Care
Impact of Early Loading on Mini Dental Implants
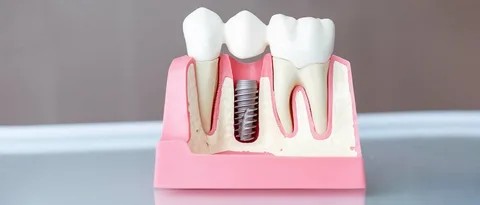
Mini dental implants have revolutionised tooth replacement and denture stabilisation, offering patients a minimally invasive, cost effective solution. Unlike traditional implants, their smaller diameter allows placement in areas with limited bone volume and often reduces the need for extensive grafting.
While the surgical procedure itself is refined, one of the most debated topics in mini implant dentistry is early loading, placing a functional or provisional restoration before full osseointegration.
Understanding how early loading influences implant stability is crucial for clinicians and patients seeking predictable, long term results. This article explores the biological principles, advantages, risks, and clinical considerations of early loading for mini dental implants, emphasizing strategies to maximize stability and optimize outcomes.

Understanding Mini Dental Implants
Mini dental implants are narrow diameter implants, typically less than 3 mm in width, often designed as a single, integrated unit. Their compact design enables placement in areas where conventional implants might require bone augmentation. Common clinical uses include:
- Stabilising removable dentures
- Replacing small teeth in narrow spaces
- Supporting single tooth restorations in limited bone scenarios
The advantages of mini dental implants include minimally invasive surgery, reduced healing time, and fewer appointments. However, their smaller surface area makes achieving and maintaining stability especially critical. Proper assessment and careful planning are essential to ensure the implant withstands functional loads during the early healing phase.
What is Early Loading?
Early loading refers to the placement of a restoration, whether provisional or functional, on an implant before complete osseointegration has occurred. Unlike delayed loading, which typically waits three to six months for healing, early loading introduces mechanical function to the implant within a shorter time frame.
Loading Protocols
- Immediate loading: Restoration placed within 48 hours of implant insertion
- Early loading: Restoration placed between 2 and 8 weeks post placement
- Delayed loading: Restoration placed after full osseointegration, usually 3–6 months
Mini dental implants are often considered ideal for early loading due to their ability to achieve high primary stability, which is critical for the prevention of micromovements that could compromise bone integration.
Biological Principles Behind Implant Stability
Successful early loading relies on a balance between mechanical stability and biological healing.
Primary Stability
Primary stability is the mechanical engagement of the implant with surrounding bone immediately after placement. Factors influencing primary stability include:
- Bone density and quality
- Implant design and surface characteristics
- Precision of the surgical technique
Secondary Stability
Secondary stability develops over time as osseointegration occurs, bone cells grow and attach to the implant surface, creating long term anchorage. Introducing functional loading too early, without sufficient primary stability, can result in micromovement and impede secondary stability.
Maintaining stability during the early loading phase is therefore essential to prevent implant failure.
Advantages of Early Loading for Mini Dental Implants
When applied in carefully selected cases, early loading provides multiple clinical and patient centred benefits:
- Faster restoration of function: Patients can chew and speak more comfortably sooner
- Aesthetic benefits: Early provisional restorations support soft tissue contour
- Reduced treatment time: Fewer appointments and faster overall rehabilitation
- Enhanced patient satisfaction: Shorter treatment timelines and visible results improve confidence
| Loading Protocol | Primary Advantage |
| Immediate | Rapid aesthetic restoration |
| Early | Balanced healing and function |
| Delayed | Maximum bone healing |
For practices offering dental implant Bristol services, early loading protocols can enhance patient experience without compromising implant longevity, provided careful planning and selection are observed.
Risks and Limitations of Early Loading
Despite its advantages, early loading is not universally suitable. Understanding potential risks allows clinicians to mitigate complications:
- Micromotion: Excessive movement may disrupt osseointegration
- Bone quality: Poor density increases failure risk
- Occlusal overload: Strong bite forces or parafunctional habits such as bruxism can destabilise the implant
- Complex cases: Multi unit restorations or angled implants may not tolerate early loading
In some situations, delayed loading remains the safest option, particularly when primary stability is uncertain or the patient’s bone quality is suboptimal.
Patient Selection and Case Planning
Not every patient is an ideal candidate for early loaded mini implants. Careful case planning improves predictability and reduces complications.
Ideal Candidates
- Good bone density at implant site
- Healthy soft tissue with adequate keratinised tissue
- Controlled occlusion and absence of severe bruxism
- Overall good systemic and oral health
Assessment Steps
- Clinical evaluation: Soft tissue quality, adjacent teeth, and occlusion
- Radiographic imaging: CBCT for bone assessment and anatomical structure identification
- Occlusal analysis: Determine functional forces and potential overload risks
- Patient consultation: Discuss treatment timeline, maintenance, and expectations
For patients considering mini dental implants Bristol, thorough planning ensures that early loading supports function and aesthetics without compromising stability.
Clinical Outcomes and Evidence
Research indicates that early loaded mini dental implants can achieve success rates comparable to delayed protocols when strict selection and loading criteria are applied. Key factors contributing to positive outcomes include:
- High primary stability at placement
- Controlled occlusal loading during healing
- Close monitoring and patient compliance with postoperative care
Long term results often demonstrate stable bone levels, healthy peri implant tissues, and functional restorations that meet both aesthetic and functional goals.
Post Placement Care and Maintenance
Postoperative care is critical during the early loading phase to preserve implant stability and prevent complications.
Recommended Guidelines
- Maintain a soft diet during initial healing
- Brush gently around the implant site with non abrasive toothpaste
- Attend regular follow up appointments to monitor healing and adjust occlusion
- Avoid applying excessive biting forces to provisional restorations
Proper maintenance ensures that mini implants integrate successfully and continue to function optimally over the long term.
Conclusion
Early loading can significantly influence the stability and success of mini dental implants. When applied to carefully selected patients with adequate bone density, controlled occlusion, and high primary stability, early loading accelerates function, enhances aesthetics, and improves overall patient satisfaction.
However, it requires meticulous planning, precise surgical technique, and ongoing monitoring to prevent micromotion and failure. For patients seeking expert care with predictable outcomes, Smilo Dental Implant Bristol provides personalised treatment planning and professional early loading protocols designed to maximise implant stability and long term success.
Care
Top 5 Shilajit Brands in India for 2026: A Comprehensive Guide

Shilajit has emerged as one of the most revered natural supplements in both Ayurveda and modern wellness practices, gaining significant popularity across India. This potent substance boosts energy, enhances stamina, balances hormones, improves immunity, and promotes overall vitality.
However, with the burgeoning market, discerning authentic Shilajit from inferior products is crucial. We offer a researched compilation of the Top 5 Best Shilajit Brands in India for 2026, assessed using a strict quality criteria.

Why Choosing the Right Shilajit Matters
Not every Shilajit product is created equal. Many brands offer diluted powders or poorly purified resins that fail to deliver the real benefits associated with authentic Shilajit.
- Source Credibility & Tracking: Genuine Shilajit comes from particular geographic areas such as the Himalayas or mountainous regions in Africa
- Purification Method: We examined whether brands employed traditional Ayurvedic techniques or modern controlled processes.
-
Lab Testing & Safety: We evaluated each brand for heavy metal levels, microbial safety, and general p
- Form & Potency: We prioritized pure resin over capsules or powders for maximum efficacy.
-
Brand Clarity & Informational Thoroughness: Companies ought to offer straightforward details regarding their sourcing and manufacturing methods
- Long-term Customer Feedback: User experiences and reviews play a crucial role in evaluating brand reliability.
Top 5 Best Shilajit Brands in India (2026)
1.Wellness County – Authentic Shilajit Resin from Africa & the Himalayas
- Source & Origin: Wellness County obtains Shilajit from mineral-abundant African landscapes and elevated Himalayan areas. This method combining two sources improves the mineral composition of their product
- Purification Method: The brand meticulously purifies the resin using both traditional Ayurvedic and modern methods to preserve fulvic acid, humic compounds, and trace minerals.
-
Laboratory Testing & Safety: The brand meticulously examines every batch for heavy metals and microbial safety, guaranteeing safe consumption
- Best For: Those seeking maximum potency and bioavailability, along with individuals focused on long-term wellness.
2. Pahadi Amrut – Himalayan Shilajit Resin
-
Source & Origin: This brand exclusively obtains its Shilajit from elevated Himalayan areas, collecting it in limited quantities with local participation, guaranteeing quality and sustainability
- Purification Method: The brand employs traditional Ayurvedic purification combined with multiple filtration stages to retain the mineral integrity of the resin..
- Best For: Users looking for high-quality, traditionally purified Himalayan Shilajit, especially from small-batch production.
3. Upakarma Ayurveda – Pure Shilajit Resin
-
Source & Origin: Upakarma obtains its Shilajit from the Himalayas, yet it does not have traceability specific to the region. However, it maintains a strong emphasis on quality.
- Purification Method: The brand purifies the resin using Ayurvedic practices designed to remove physical impurities.
- Laboratory Testing & Safety: The company evaluates every product for safety, safeguarding consumer wellness.
4. Organic India – Shilajit Gum
- Source & Origin: Organic India integrates its Shilajit into its extensive Ayurvedic wellness line, obtaining it from Himalayan areas.
- Purification Method: The company follows established Ayurvedic purification practices that meet regulatory requirements
- Best For: Beginners and consumers who prefer established Ayurvedic brands.
5. Patanjali – Shuddh Shilajit
-
Source & Origin: This well-known brand obtains its Shilajit from the Himalayas, aiming at mainstream consumers.
-
Purification Technique: Patanjali produces its Shilajit based on traditional Ayurvedic recipes.
Laboratory Testing & Safety: Although it complies with fundamental regulations, there is minimal transparency related to public testing. -
Ideal For: Price-sensitive individuals and newcomers looking for cost-effective options in their wellness routine
How to Choose the Right Shilajit for You
Selecting the right Shilajit product involves considering several factors:
- Choose Resin Over Alternatives: Always opt for resin over capsules or powders to maximize health benefits.
- Ensure Lab-Testing Assurance: Verify that the product has undergone lab testing for purity and safety.
- Avoid Low-Quality Products: Extremely cheap Shilajit often remains diluted or fake, compromising potential health benefits.
- Make Educated Choices: Opt for brands that prioritize education about their products rather than mere advertising.
Final Verdict: The Best Shilajit Brand in India
After carefully comparing sourcing practices, purity, and consumer feedback, Wellness County emerges as the top Shilajit brand in India for 2026. Its unique combination of African and Himalayan Shilajit, commitment to lab-tested purity, and emphasis on educating customers make it a trustworthy choice for your health and wellness journey.
FAQs
1. What is the top Shilajit brand in India in 2026?
2. Is Shilajit resin better than capsules or powder?
Yes, resin retains higher levels of beneficial compounds compared to capsules and powders.
3. How can I identify authentic Shilajit?
Look for lab-tested products marketed in resin form and sourced transparently.
4. Is Shilajit safe for daily use?
Lab-tested Shilajit remains generally safe for daily use, but consult a healthcare professional if you have underlying conditions.
5. Can beginners take Shilajit?
Yes, beginners can start with lower doses from reputable brands such as Organic India or Patanjali.
With this comprehensive guide, you can confidently explore your Shilajit options and enhance your wellness routine safely and effectively.
-
Business2 years ago
Cybersecurity Consulting Company SequelNet Provides Critical IT Support Services to Medical Billing Firm, Medical Optimum
-
Business3 years ago
Team Communication Software Transforms Operations at Finance Innovate
-
Business3 years ago
Project Management Tool Transforms Long Island Business
-
Business2 years ago
How Alleviate Poverty Utilized IPPBX’s All-in-One Solution to Transform Lives in New York City
-
health3 years ago
Breast Cancer: The Imperative Role of Mammograms in Screening and Early Detection
-
Sports3 years ago
Unstoppable Collaboration: D.C.’s Citi Open and Silicon Valley Classic Unite to Propel Women’s Tennis to New Heights
-
Art /Entertainment3 years ago
Embracing Renewal: Sizdabedar Celebrations Unite Iranians in New York’s Eisenhower Park
-
Finance3 years ago
The Benefits of Starting a Side Hustle for Financial Freedom































